The following is the information provided in the program and some
screen shots.
H3D
A representation of the atomic orbitals of the hydrogen atom.
Stuart Prescott 1996
================================================
Using the program (and a bit of Quantum theory):
Firstly, select the orbital which you wish to view. This may be
done by clicking on the icon corresponding to the name of the
orbital.
The graphs of the wave (or density) functions passing through
the maximum in the other two variables are shown. Here,
theta is the azimuth (angle with the Z-axis) and phi is the co-
latitude (the angle from the X-axis in the XY-plane).
The surface drawn in the 3D drawing of the orbital is a surface
of constant wave (or density) function. The value of this
surface level is shown above the plot and may be changed by
dragging the horizontal bars that appear on the graph. (Note
that a surface level of zero cannot generate a surface, so the
minimum surface level is 10e-7)
The view of the orbital may be rotated using the ArcBall
which appears in the top left hand corner of the window.
To use the ArcBall, imagine that the circle represents a
sphere, and then use the mouse to 'drag' the sphere around.
The large triangle drawn indicates the axis about which
the rotation will be performed, and the length of the arc
corresponds to how much the rotation will be.
To return the orbital to the original viewing position, click
on the"Reset View" button.To select a new orbital to view,
click on the "Select Orbital" button.
The "Show Wavefunction"/"Show Density" button changes
the function displayed by the program. The wave function
is the representation of Schrodinger's equation, the green
lobes are where the wavefunction is negative and the red
lobes are where is is positive. The density function shows
the probability of finding an electron at any point in space,
and is the square of the wave function.
================================================
Advanced Features:
For those who aren't happy with the quality of the images
produced by the interpolation (or for those with plenty of
time on their hands), the size of the interpolation cell
can be changed.
By clicking with the right mouse button in the vicinity of
the 3D orbitals, a window is produced allowing the choice of
interpolation cell sizes.
The smaller the cell size, the more memory is required to do
the drawing, and the longer will be the time taken to draw
the 3D representation, but the quality of the image will be
higher. On some systems, the memory requirement of running
at a higher resolution will cause memory management faults.
On some orbitals striped or checkerboard patterns may be
observed. These patterns are due to the interpolation.
Basically, it comes down to a speed v accuracy issue, and
speed won.
A cell size of 5 pixels is the default (and recommended) size.
================================================
About the development:
This program was written by Stuart Prescott in 1996 as a
project for the First Year Talented Student Program within
the Basser Department of Computer Science at the
University of Sydney. This project was supervised by Dr Ian
Parkin of the same department. It is both a first program
in C++, and a first computer graphics application.
The calculation engine used by the program was taken from
the "H2 Workshop Program" written in FORTRAN by George
Bacskay and Jeff Reimers, Department of Physical and
Theoretical Chemistry, University of Sydney.
The ArcBall was developed by Ken Shoemake, Computer
Graphics Laboratory, University of Pennsylvania, as
an intuitive, easy-to-use way of rotating objects using
the computer mouse.
The graphics routines were written using Libsx (The
Simple X library) version 1.1 - a friendly overlay to the
Athena Widget Set. Libsx was developed by Dominic
Giamapolo (dbg@sgi.com) and is available from
"ftp.x.org/contrib/libraries/libsx.tar.Z"
================================================
Copyright (c) Stuart Prescott MCMXCVI
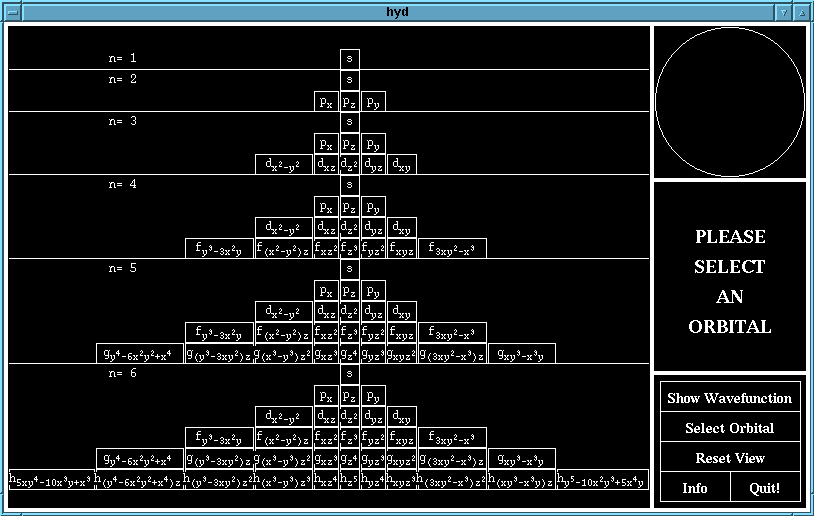
The main selection screen
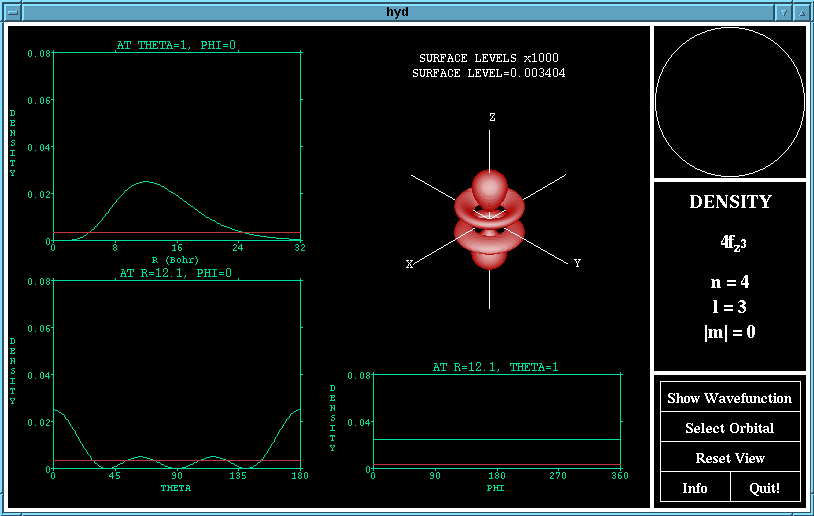
The density function of the 4fz^3 orbital
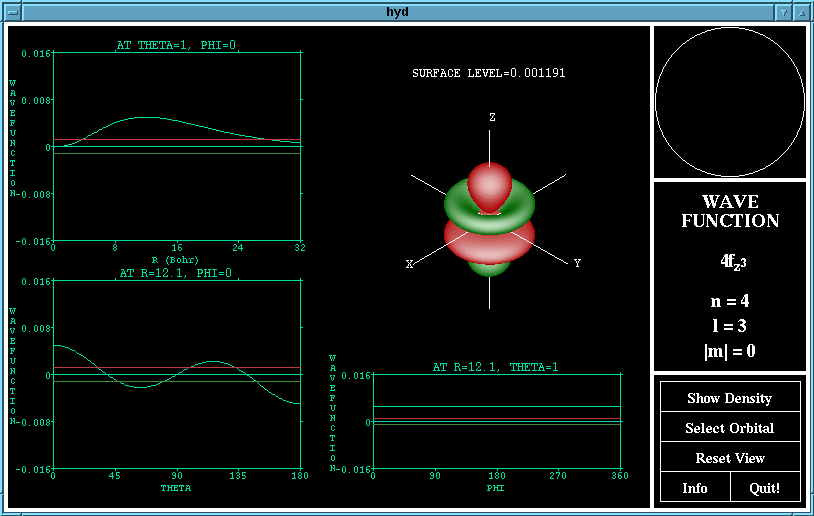
The wave function of the 4fz^3 orbital
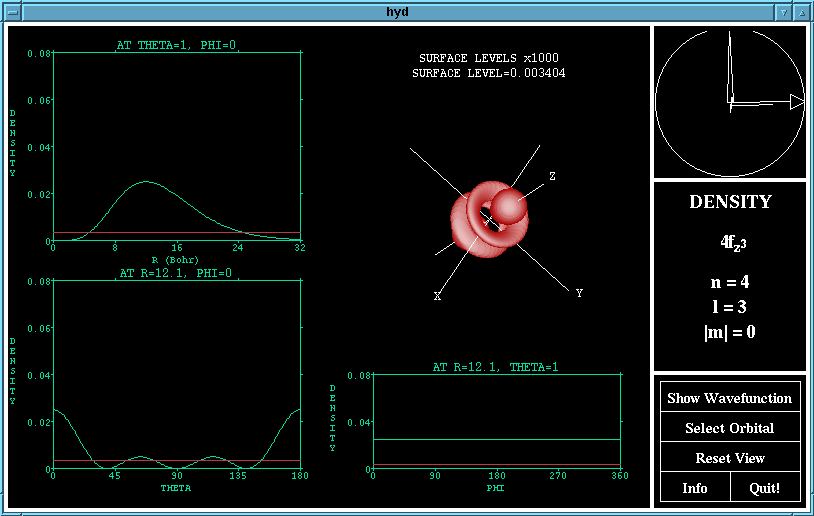
A rotation of the density function of the 4fz^3
orbital
Last edited: Wednesday June 23, 2004
Copyright © 1996-2014 Stuart Prescott